
Please note: Some images on this page are not work-safe.
The information on this page allows one to infer how masculine or feminine different women are, and should be used to compare high-fashion models with glamour models.
Men develop under higher androgen levels than women. Women develop under higher estrogen levels than men. Both androgens and estrogens affect various skeletal structures and soft tissues in different ways. Therefore, an examination of overall physical appearance allows one to compare the ratios of testosterone (a major androgen) to estradiol (a major estrogen) that people of the same ethnic group have developed under.
Some of the major physique differences between men and women are well-known and are not addressed here. For example, a woman with broad shoulders and a narrow pelvis is more masculinized than the typical woman. The discussion below mostly focuses on facial features and pelvic/torso skeletal structure. Please note that since sex hormones are only partly responsible for trait variation, it is necessary to consider a cluster of traits known to be influenced by sex hormones to assess the masculinity-femininity of a person. A follow-up addressing masculinity-femininity in men can be found here.
The skull
Fig 1 shows variation in [European] skull shape resulting from sex differences, after removing the size-differences factor. The middle skull (top row) represents the average of men and women; the skulls to its left and right represent feminization (effect of estrogens) and masculinization (effect of androgens), respectively. Note the two-dimensional matrix in the background of the middle skull; the directionality of its deformation in the background of the other two skulls represents the effects of sex hormones.
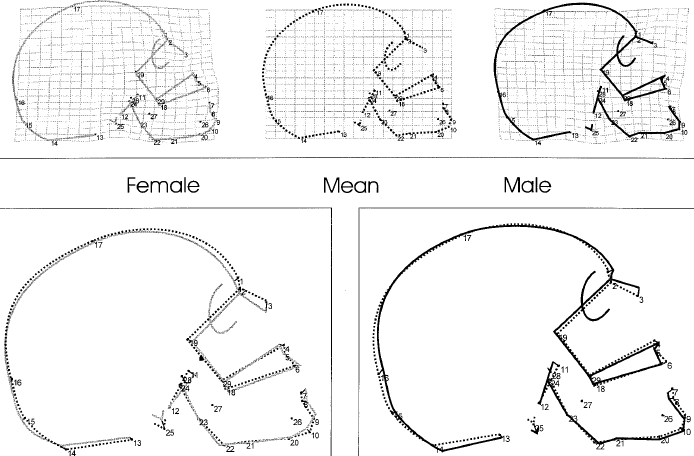
Fig 1: Change in skull shape as a function of sex, assessed via geometric morphometrics.(1) The first row shows transformation grids from mean centroid size into a female and into a male. The bottom row shows corresponding generalized least-square superimpositions. "In males, the nasoglabellar profile [region around points 1 and 2 in the figure] becomes curved and projecting, and the subnasal profile rotates clockwise. The nasal roof is elevated, and the nasal floor shifts downwards. The outer table of the occipital clivus rotates counterclockwise. The chin is lowered, and the gonial region gets strongly curved [the gonion is marked at point 22]. At the occipital bone in males [back of the skull], the squamous proportion dominates over the nuchal area, and the whole basi- and neurocranium is deformed globularly."(3) The numbers are defined as follows: 1, Glabella; 2, Nasion; 3, Rhinion; 4, Anterior nasal spine; 5, A-point (deepest point on the curvature of the alveolar clivus in the median sagittal plane); 6, Prosthion; 7, Infradentale; 8, B-point (deepest point at the mandibular symphysis curvature [between landmark 7 and landmark 9]); 9, Menton; 10, Gnathion; 11, Vomero-sphenoid junction; 12, Basion; 13, Ophistion; 14, Inion; 15, Ophistocranion; 16, Lambda; 17, Bregma; 18, R. alveolar point (right posterior limit of the maxillary alveolar arc at the pterygo-alveolar suture); 19, R. optic canal point (most superior, medial, and anterior points of the optic canal [superior border of the posterior face]); 20, R. inf. basal border; 21, R. preangular incisure; 22, R. gonion; 23, R. ramus flexion; 24, R. condylion; 25, R. mastoid tip (most distal point at the mastoid process); 26, R. mental foramen (anterior limit); 27, R. lingula mandibulae (antero-lateral base); 28, R. foramen ovale (postero-lateral corner); 29, Posterior nasal spine.(1)
Fig 2 summarizes the finds obtained in Fig 1.
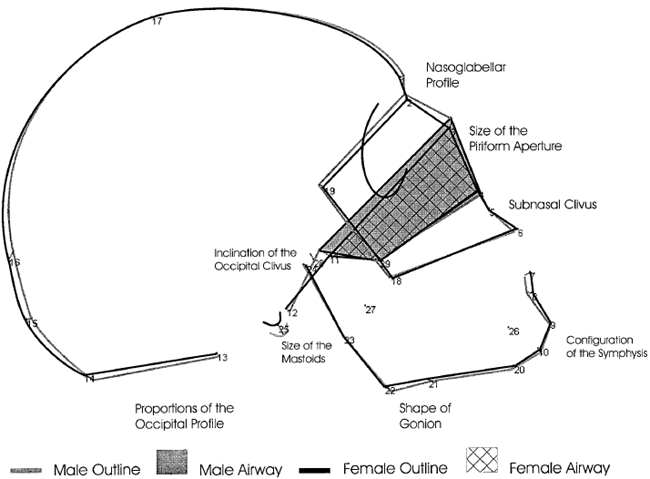
Fig 2: Change in skull shape as a function of masculinization and feminization.(1)
Fig 3 sums up some elements of cranial shape variation resulting from masculinization and feminization, and includes some of the changes not addressed in Fig 1. The technical terms in Fig 3 are explained below it.
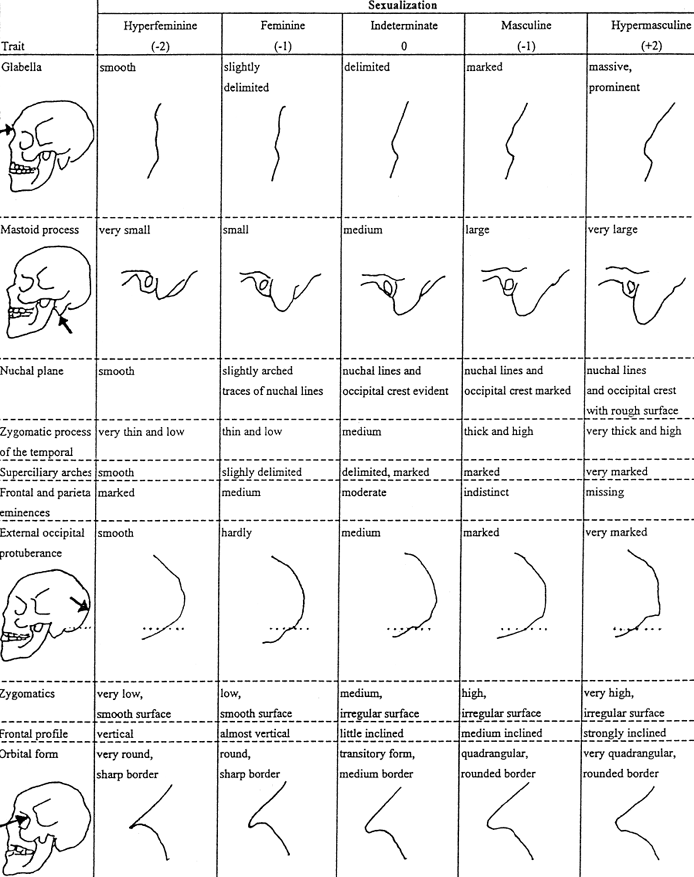
Fig 3: Cranial shape variation related to masculinization and feminization.(2-4)
In Fig 3, changes related to the mastoid process, nuchal plane (back of the skull at the nape of the neck) and external occipital protuberance are not relevant for a visual comparison based on photographs of real people because hair would typically hide the differences. Fig 3 also mentions shape changes in the superciliary and zygomatic arches, which are explained next. Notice the spot marked as the glabella in Figure 3. The superciliary arches are the arches on the forehead, right above the eyebrows, which extend sideways on both sides of the glabella. The zygomatics are the cheekbones, shown in orange in Fig 4, which also depicts the cross-hatched bony portion known as the zygomatic arch.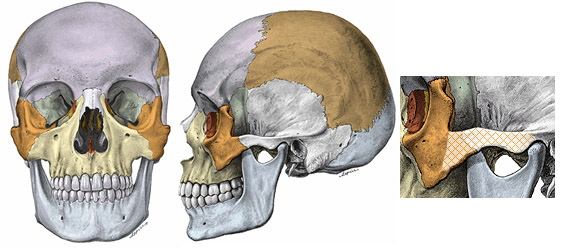
Fig 4: Zygomatic bones, also known as cheekbones (orange) and the zygomatic arch (cross-hatched portion). The cheekbones are higher in males, lower in females; and the zygomatic arches are higher and thicker in males, lower and thinner in females.(3, 4) Illustrations taken from Sobotta Atlas of Human Anatomy.
Fig 5 shows real-life examples of the skull of a feminine woman (left) and that of a masculine man.
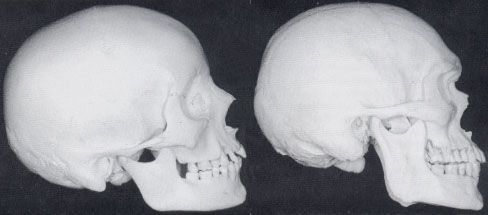
Fig 5: The skulls of a feminine woman (left) and a masculine man.
Fig 6 addresses change in [European] skull shape as a function of size. Note that increasing face size sharpens the gonial angle (at point 22), giving it a masculine appearance.
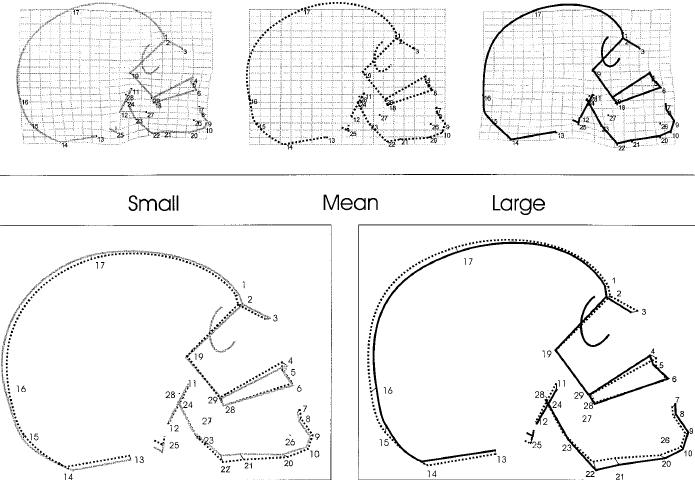
Fig 6: Change in face shape as a function of size, assessed via geometric morphometrics.(1) The first row shows transformation grids from mean centroid size into a small individual and into a large one. The bottom row shows corresponding generalized least-square superimpositions. "With increasing centroid size [face size], the subnasal clivus becomes enlarged and rotates counterclockwise; the increment of the mandibular ramus [vertical portion of lower jaw bone] height leads to a more pronounced angulation at gonion [point 22]; the antero-posterior dimensions of the splanchnocranium are reduced; the face is expanded supero-inferiorly; the face appears relatively larger than the neurocranium; and the braincase becomes more globular."(1) See the legend of Fig 1 for the definitions of the numbers.
There are additional changes in facial features related to sex hormones; these changes are addressed after controlling for size: the eyebrows are thicker and placed lower on the face, i.e., closer to the eyes, in males; the face is broader in the female; the distance between the eyes is greater in the female; and the chin is longer and squared in the male (Fig 7a).
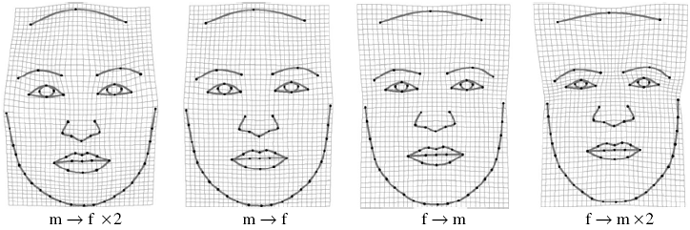
Fig 7a: Face outline variation resulting from masculinization and feminization (front view, assessed via geometric morphometrics); f = female, m = male.(5)
In Fig 7b, the middle column represents the male-female average (northwest Europeans); the left and right columns represent equal displacements in the male and female direction, respectively. The differences between the left and right columns represent an approximately three-fold exaggeration of average male-female differences.

Fig 7b. Sexual dimorphism in face shape; see text for details.(6)
Figure 7c describes how a female face is transformed by increased feminization. The left column shows the directionality of displacement of parts of the face; the blue part moves inward and the red part outwards; stronger color implies that the directionality of the displacement is closer to right angles to the surface. The middle column shows the magnitude of the displacement; stronger color implies greater magnitude of change; the color coding at each point is in 33% percentiles. The right column shows change in [local] surface area; red implies increase and blue decrease; color coding at each point is in 33% percentiles, with darker colors implying the upper percentiles.

Fig 7c. Face shape change resulting from feminization of a female face; see text for details.(6)
Increasing feminization causes the following important facial changes:(6)
(key in a standing person: anterior = toward front, posterior = toward back, superior = above, inferior = below, medial = toward midline, coronal plane = vertical plane dividing body into anterior-posterior parts)
- The upper lip and philtrum area is displaced posteriorly and superiorly, and reduced in area, especially in the middle part.
- The nose becomes narrower along its length, reduces in area and is displaced posteriorly. The tip of the nose moves posteriorly, making the nose become relatively shorter, and the tip is also displaced superiorly, which turns up the tip of the nose.
- The cheeks expand in area and are displaced outwards.
- The chin reduces in area and is displaced superiorly and posteriorly. The angle of the mandible is displaced toward the middle of the face, and the mandible is reduced in relative length.
- The inferior ear attachment is displaced medially whereas the superior ear attachment is displaced inferiorly, leading to an overall rotation of this area in the coronal plane.
- "The superior medial orbital margins are displaced superiorly and posteriorly whereas the superior lateral margins are displaced laterally; the lateral margins are displaced laterally."
- The medial forehead is displaced anteriorly and expands laterally; the lateral aspects are displaced anteriorly without lateral expansion.
In Fig 8, the rank-ordering along the lines of masculinity-femininity should be obvious, and the reader should now be able to justify the rank-ordering with some precision. Reflecting the global effects of sex hormones, the rank-ordering of the overall physique of these three women along the lines of masculinity-femininity dovetails with that of their faces.
![]()


Fig 8: An example of masculine-to-feminine face shape variation. The leftmost picture in the first row has been taken from the cover of "Down came the rain" by Brooke Shields and the pictures of the other women have been taken from Twistys (adult site).
Torso/Pelvic structure
Controlling for height, women have a smaller rib cage than men and a wider pelvis. Fig 9 shows the importance of a feminine (small) rib cage in achieving an hourglass figure, which the woman shown cannot achieve, even with breast implants.
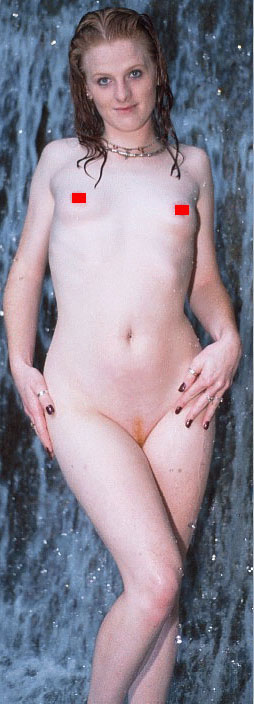
Fig 9: A masculinized woman with a large rib cage.
In Fig 10, the masculinized woman shown does not have an hourglass figure because of both a narrow pelvis and a broad rib cage, and once again, even breast implants are not going to help this woman achieve an hourglass figure.

Fig 10: A non-overweight, masculinized woman. Note the posing of the pelvic region. Glamour models with a narrow pelvis typically pose in the twisted manner shown in order to make the male-like shape of their pelvic structure less obvious. Picture taken from Karupspc (adult site).
Fig 11 contrasts a glamour model (left) with a high-fashion model (right). The high-fashion model shown is clearly among the more feminine ones compared to the norm. Given that a number of feminine women refuse to pose naked and that the selection of glamour models in some major outlets such as Playboy magazine has been influenced by the high status of high-fashion models, some authors(7) have pointed out that with an average waist-to-hip ratio (WHR) of 0.71, fashion models have an hourglass figure given that Playboy models have an average WHR of 0.68!

Fig 11: Contrast rib cage relative size between the glamour model (left) and the high-fashion model.
WHRs of 0.71 and 0.68 may not sound that different, but see the discussion of the data provided by Tovee et al. in Table 1 here. On the other hand, one look at Fig 12 suffices to show what a feminine WHR is supposed to look like; very feminine WHRs are in the 0.60-0.65 range. Note that the feminine women in Fig 12 have smaller breasts than both the glamour model and the high-fashion model in Fig 11, yet present a more feminine look overall. Furthermore, contrast the masculine arm length of the high-fashion model in Fig 11 (a typical example) with the feminine arm lengths of the glamour models in Fig 12.

Fig 12: A good illustration of what feminine waist and hip proportions are supposed to look like.
By now, the importance of skeletal structure -- as it is shaped by sex hormones -- in the appearance of a feminine, hourglass figure should be obvious. Fig 13 should help make this point clearer as it shows a slender, small-breasted woman that nevertheless has a feminine pelvic region and torso, thanks to a pelvis that is wide enough for an attractive woman and a small rib cage.

Fig 13: A slender woman with small breasts that nevertheless has feminine pelvic and torso proportions due to a feminine skeletal structure. Picture taken from Karupspc (adult site).
Fig 14 addresses sexual dimorphism in pelvic structure. In a standing person, the female pelvis is broader when viewed from the front or side and its vertical length is smaller.
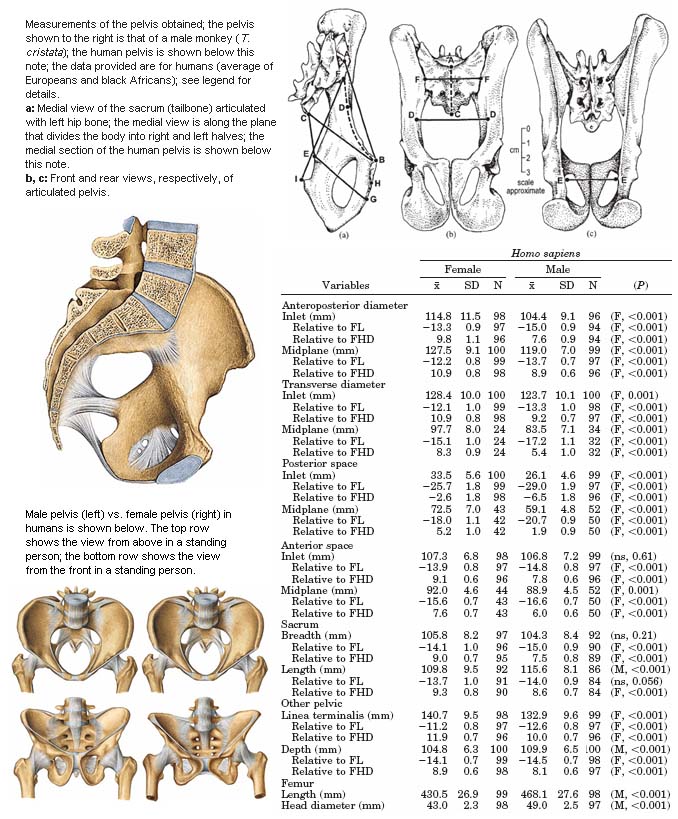
Fig 14: Sexual dimorphism in the pelvis. The measures provided are referenced with respect to FL (femur length; the femur is the thigh bone) and FHD (femur head diameter) and defined in terms of the points marked [in 3 figures on top right] as follows.(8) “Anteroposterior diameters: inlet (a, A–B), sacral promontory to dorsomedial border of superior aspect of pubic body; midplane (a, B–C), dorsomedial border of superior aspect of pubic body to lower sacral vertebra. Anteroposterior diameter of midplane was taken [in humans] from transverse line between fourth and fifth sacral vertebra to dorsomedial border of inferior aspect of primary pubic symphysis. Transverse diameters: inlet (b, D–D), maximum distance between lineae terminales, with this diameter being aligned visually to be perpendicular to anteroposterior diameter of inlet; midplane (c, E–E), distance between ischial spines. Posterior spaces: inlet (a, D–F), curved length along linea terminalis from transverse diameter of inlet to intersection with auricular surface of ilium; midplane (a, C–E), point on sacral vertebra used in measurement of anteroposterior diameter of midplane to ischial spine. Anterior spaces: inlet (a, B–D), curved length along linea terminalis from dorsomedial border of superior aspect of pubic body to transverse diameter of inlet; midplane (a, E–H), ischial spine to dorsomedial border of inferior aspect of primary pubic symphysis (a, H). Sacrum: breadth (b, F–F), straight distance across ventral aspect of sacrum where sacrum met linea terminalis when pelvis was articulated; length (b, A–C), curved length from sacral promontory to point on sacral vertebra used in measurement of anteroposterior diameter of midplane; for H. sapiens, sacral length was from sacral promontory to inferior border of fifth sacral vertebra. Other measures: linea terminalis (a, B–F), curved length from dorsomedial border of superior aspect of pubic body to auricular surface of ilium; depth (a, F–I), intersection of linea terminalis and auricular surface of ilium to inner margin on dorsal aspect of ischial tuberosity.”(8) The F and M statistics are reported for Student’s t-test and Wilcoxon-Mann-Whitney test, respectively. The color illustrations have been taken from Sobotta Atlas of Human Anatomy.
Fig 15 shows an example of a feminine backside. Figures 16 and 17 illustrate the folly of judging femininity via breast size alone. As seen in Fig 16, it is fairly common to come across large-breasted women with backsides that are flatter than the feminine norm. Similarly, in Fig 17, the obese women not only have massive breasts but also a large pelvis, yet their backsides are flatter than the feminine norm. The women shown in Figures 16 and 17 have sufficient estradiol to produce large breasts, but their testosterone-to-estradiol ratio is elevated, and development under above-average levels of testosterone is responsible for a masculinized WHR and a masculinized pelvis in these women, and the elevated testosterone levels are also correlated with excessive abdominal fat in the women shown in Fig 17. See here for the relevant citations concerning elevated testosterone levels in obese women or women with an elevated WHR and also evidence that large breasts are diagnostic of femininity if accompanied by a small waist and -- by extrapolation -- a feminine backside [given the global effects of sex hormones]. The feminine protrusion of the backside in normal women is mainly a function of pelvic bone structure, although in some African and aboriginal populations, fat mass can make a notable contribution to the prominence of the backside in women.

Fig 15: An example of a feminine backside. Picture taken from FTV girls (adult site).

Fig 16: Two examples of women with large breasts that nevertheless have backsides that are flatter than the feminine norm. Pictures taken from Score magazine (adult site).

Fig 17: Two examples of obese women with large breasts, yet backsides that are flatter than the feminine norm. Pictures taken from Score magazine (adult site).
Another example that should help one appreciate the importance of judging masculinity-femininity from overall appearance is shown below. Which of the two women in the following picture looks more feminine?
Fig 18. Melissa from Teen Stars Magazine (left) and Ludmila from Domai; click for larger image.
Now look at the following two pictures of the women in Fig 18 and answer who is more feminine.

Fig 19. Ludmila from Domai (left) and Melissa from Teen Stars Magazine; links lead to adult sites.

Fig 20. Ludmila from Domai (left) and Melissa from Teen Stars Magazine; links lead to adult sites.
In Fig 18, the small breasts and large nose of the light woman suggest reduced feminization and greater masculinization, respectively, but the dark woman has broader shoulders, narrower hips, a smaller backside, a more masculine waist-to-hip ratio, a stronger chin and more masculine hands and feet (not clear in the pictures shown). Thus, the appropriate conclusion is that that the light woman is more feminine, and her large nose and small breasts do not represent greater masculinization and lesser feminization, respectively, but other factors, instead.
On the other hand, when one comes across physiques like those shown in Fig 21, there is little doubt as to where they stand on the masculine-feminine scale.

Fig 21. Dasha from Model flats (left) and Elkie from Simonscans; links lead to adult sites.
References
- Rosas, A., and Bastir, M., Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex, Am J Phys Anthropol, 117, 236 (2002).
- Walrath, D. E., Turner, P., and Bruzek, J., Reliability test of the visual assessment of cranial traits for sex determination, Am J Phys Anthropol, 125, 132 (2004).
- Buikstra, J., Ubelaker, D., and Aftandilian, D. A., Standards for data collection from human skeletal remains, Arkansas Archaeological Survey, Fayetteville, AK (1994).
- Ferembach, D., Schwidetzky, I., and Stloukal, M., Recommendations for age and sex diagnoses of skeletons, J Hum Evol, 9, 517 (1980).
- Fink, B., Grammer, K., Mitteroecker, P., Gunz, P., Schaefer, K., Bookstein, F. L., and Manning, J. T., Second to fourth digit ratio and face shape, Proc R Soc B, 272, 1995 (2005).
- Hennessy, R. J., McLearie, S., Kinsella, A., Waddington, J. L. Facial surface analysis by 3D laser scanning and geometric morphometrics in relation to sexual dimorphism in cerebral--craniofacial morphogenesis and cognitive function, J Anat, 207, 283 (2005).
- Tovee, M. J., Mason, S. M., Emery, J. L., McCluskey, S. E., and Cohen-Tovee, E. M., Supermodels: stick insects or hourglasses? Lancet, 350, 1474 (1997).
- Tague, R. G., Big-bodied males help us recognize that females have big pelves, Am J Phys Anthropol, 127, 392 (2005).
